The mDOT Center
Transforming health and wellness via temporally-precise mHealth interventions






mDOT@MD2K.org
901.678.1526
901.678.1526








Collaborating Investigators:
Dr. David Wetter, University of Utah
Funding Status:
NIH/NCI
05/15/24 – 04/30/30
Associated with:
Lung cancer is the leading cause of cancer death in the U.S., with smoking causing about 80% of cases. Despite effective, recommended annual low-dose CT screening (LCS), only 6.5% of eligible individuals were screened in 2020, with significant demographic and socioeconomic disparities. LungSMART Utah seeks to improve equitable LCS uptake in low-resource settings through a two-phase SMART trial in Utah Community Health Centers, which serve over 160,000 predominantly Latino, rural, and uninsured patients. Using a Population Health Management approach, Phase 1 employs telehealth tools for eligibility assessment, shared decision-making, and referral, while Phase 2 tests interventions addressing barriers to completing screening. A centralized Hub and mobile technologies – including support for patients with only cell phone access – deliver culturally and linguistically tailored care in real-world settings. This project will provide critical evidence to support scalable, equity-focused LCS implementation nationwide.
TR&D1 pushes its optimized screening eligibility algorithms using predictive modeling for personalized LCS outreach and improving patient engagement strategies through AI-driven adaptive messaging for Shared Decision Making, strengthening LungSMART’s ability to deliver data-driven, adaptive, and scalable engagement interventions.
PI Wetter has collaborated with TR&D1 on developing and piloting adaptive mobile interventions aimed at reducing tobacco use and promoting preventive care in assorted populations. This work focused on integrating behavioral data and social determinants of health into real-time decision-making models and helped establish scalable frameworks for digital health interventions in low-resource settings – foundational efforts that directly inform the current LungSMART Utah project.
ACM on Interactive, Mobile, Wearable, and Ubiquitous Technologies (IMWUT)
September 7, 2022
behavioral intervention, human-centered computing, risk prediction, smoking cessation, ubiquitous and mobile computing design and evaluation methods, wearable sensors
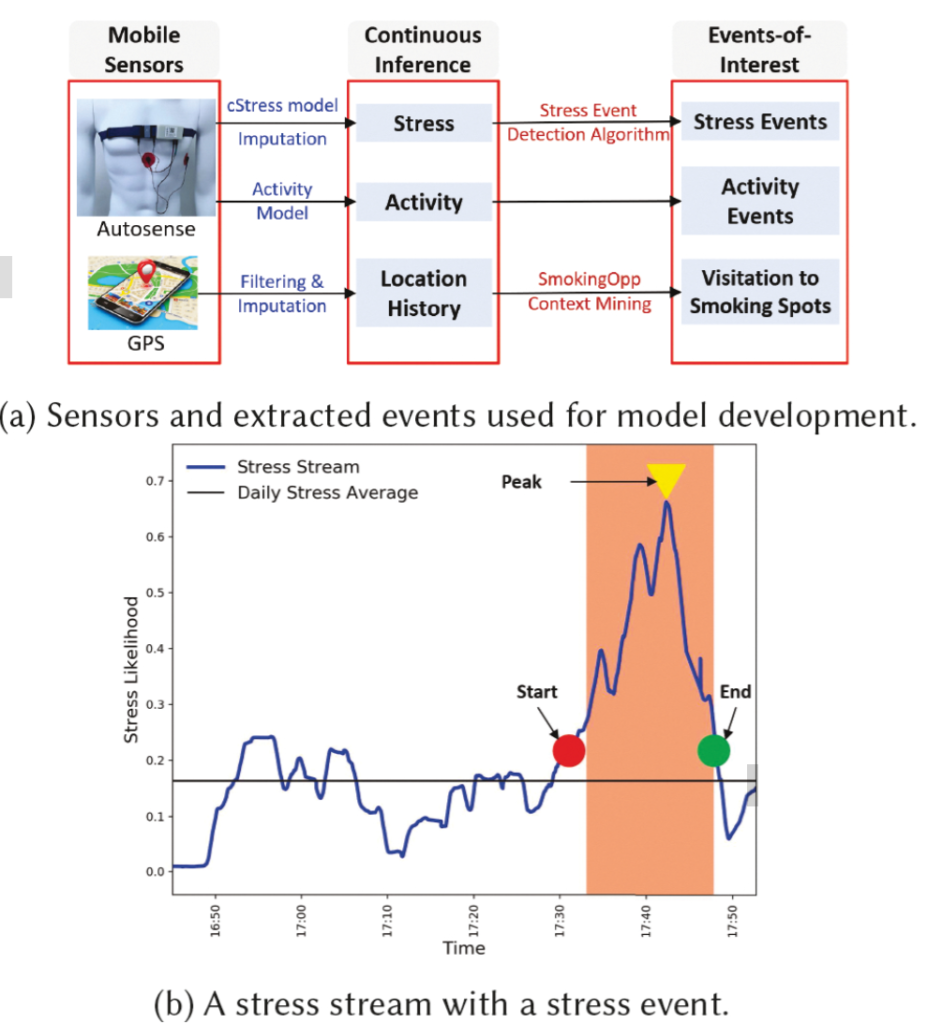
Passive detection of risk factors (that may influence unhealthy or adverse behaviors) via wearable and mobile sensors has created new opportunities to improve the effectiveness of behavioral interventions. A key goal is to find opportune moments for intervention by passively detecting rising risk of an imminent adverse behavior. But, it has been difficult due to substantial noise in the data collected by sensors in the natural environment and a lack of reliable label assignment of low- and high-risk states to the continuous stream of sensor data. In this paper, we propose an event-based encoding of sensor data to reduce the effect of noises and then present an approach to efficiently model the historical influence of recent and past sensor-derived contexts on the likelihood of an adverse behavior. Next, to circumvent the lack of any confirmed negative labels (i.e., time periods with no high-risk moment), and only a few positive labels (i.e., detected adverse behavior), we propose a new loss function. We use 1,012 days of sensor and self-report data collected from 92 participants in a smoking cessation field study to train deep learning models to produce a continuous risk estimate for the likelihood of an impending smoking lapse. The risk dynamics produced by the model show that risk peaks an average of 44 minutes before a lapse. Simulations on field study data show that using our model can create intervention opportunities for 85% of lapses with 5.5 interventions per day.
We present a model for identifying ideal moments for intervention by passively detecting risk of an imminent adverse behavior.
Sy-Miin Chow, Inbal Nahum-Shani, Justin Baker, Donna Spruijt-Metz, Nicholas Allen, Randy Auerbach, Genevieve Dunton, Naomi Friedman, Stephen Intille, Predrag Klasnja, Benjamin Marlin, Matthew Nock, Scott Rauch, Misha Pavel, Scott Vrieze, David Wetter, Evan Kleiman, Timothy Brick, Heather Perry, Dana Wolff-Hughes
Translational Behavioral Medicine, Volume 13, Issue 1, January 2023, Pages 7–16
November 23, 2022
EMA, health behavior changes, ILHBN, location, sensor
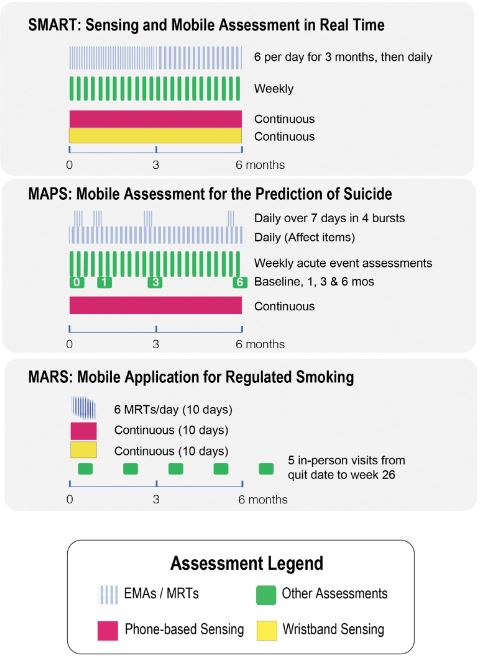
The ILHBN is funded by the National Institutes of Health to collaboratively study the interactive dynamics of behavior, health, and the environment using Intensive Longitudinal Data (ILD) to (a) understand and intervene on behavior and health and (b) develop new analytic methods to innovate behavioral theories and interventions. The heterogenous study designs, populations, and measurement protocols adopted by the seven studies within the ILHBN created practical challenges, but also unprecedented opportunities to capitalize on data harmonization to provide comparable views of data from different studies, enhance the quality and utility of expensive and hard-won ILD, and amplify scientific yield. The purpose of this article is to provide a brief report of the challenges, opportunities, and solutions from some of the ILHBN’s cross-study data harmonization efforts. We review the process through which harmonization challenges and opportunities motivated the development of tools and collection of metadata within the ILHBN. A variety of strategies have been adopted within the ILHBN to facilitate harmonization of ecological momentary assessment, location, accelerometer, and participant engagement data while preserving theory-driven heterogeneity and data privacy considerations. Several tools have been developed by the ILHBN to resolve challenges in integrating ILD across multiple data streams and time scales both within and across studies. Harmonization of distinct longitudinal measures, measurement tools, and sampling rates across studies is challenging, but also opens up new opportunities to address cross-cutting scientific themes of interest.
The article shares insights, challenges, opportunities, and solutions from harmonizing intensive longitudinal data within the ILHBN, providing tools and recommendations for future data harmonization efforts.
Addictive Behaviors, Volume 136, p.107467
January 2023
just-in-time adaptive intervention, micro-randomized trial, mindfulness; smoking cessation; mHealth
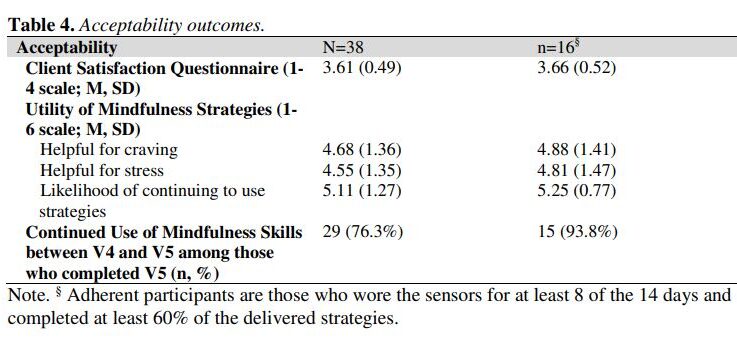
Smoking cessation treatments that are easily accessible and deliver intervention content at vulnerable moments (e.g., high negative affect) have great potential to impact tobacco abstinence. The current study examined the feasibility and acceptability of a multi-component Just-In-Time Adaptive Intervention (JITAI) for smoking cessation. Daily smokers interested in quitting were consented to participate in a 6-week cessation study. Visit 1 occurred 4 days pre-quit, Visit 2 was on the quit day, Visit 3 occurred 3 days post-quit, Visit 4 was 10 days post-quit, and Visit 5 was 28 days post-quit. During the first 2 weeks (Visits 1-4), the JITAI delivered brief mindfulness/motivational strategies via smartphone in real-time based on negative affect or smoking behavior detected by wearable sensors. Participants also attended 5 in-person visits, where brief cessation counseling (Visits 1-4) and nicotine replacement therapy (Visits 2-5) were provided. Outcomes were feasibility and acceptability; biochemically-confirmed abstinence was also measured. Participants (N = 43) were 58.1 % female (AgeMean = 49.1, mean cigarettes per day = 15.4). Retention through follow-up was high (83.7 %). For participants with available data (n = 38), 24 (63 %) met the benchmark for sensor wearing, among whom 16 (67 %) completed at least 60 % of strategies. Perceived ease of wearing sensors (Mean = 5.1 out of 6) and treatment satisfaction (Mean = 3.6 out of 4) were high. Biochemically-confirmed abstinence was 34 % at Visit 4 and 21 % at Visit 5. Overall, the feasibility of this novel multi-component intervention for smoking cessation was mixed but acceptability was high. Future studies with improved technology will decrease participant burden and better detect key intervention moments.
The study assessed the feasibility and acceptability of a multi-component Just-In-Time Adaptive Intervention (JITAI) for smoking cessation, utilizing smartphone-delivered mindfulness/motivational strategies based on real-time negative affect or smoking behavior detected by wearable sensors. Participants showed high retention (83.7%) and reported high satisfaction with the intervention, but the feasibility was mixed.
Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies
mobile health, context, smoking cessation, intervention, GPS traces
March 2020
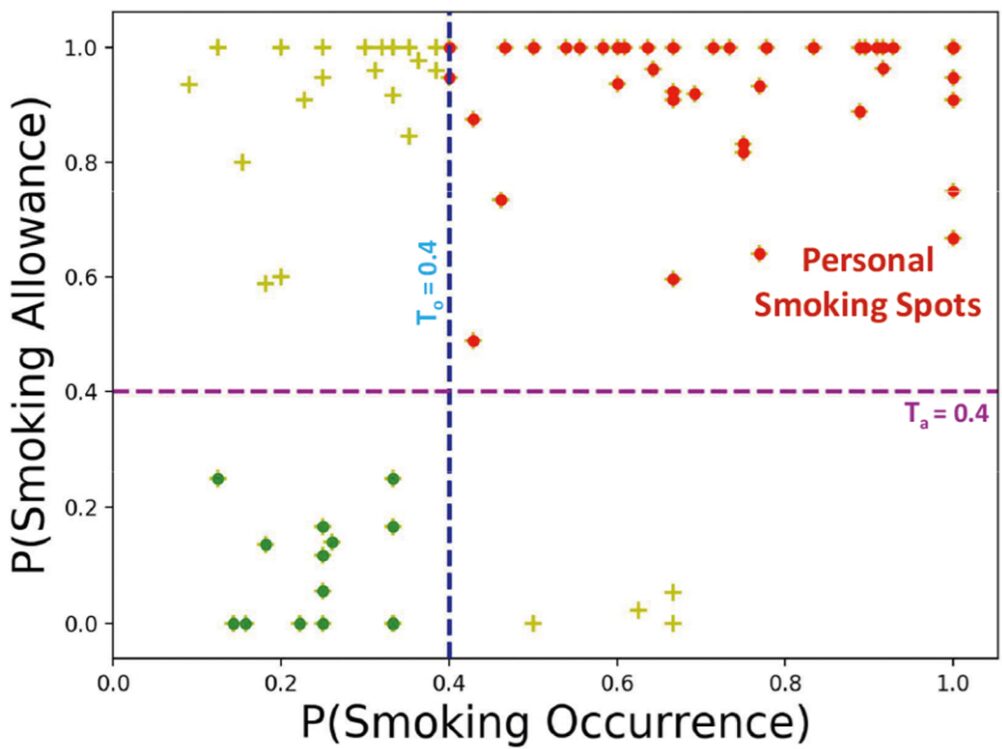
Context plays a key role in impulsive adverse behaviors such as fights, suicide attempts, binge-drinking, and smoking lapse. Several contexts dissuade such behaviors, but some may trigger adverse impulsive behaviors. We define these latter contexts as ‘opportunity’ contexts, as their passive detection from sensors can be used to deliver context-sensitive interventions. In this paper, we define the general concept of ‘opportunity’ contexts and apply it to the case of smoking cessation. We operationalize the smoking ‘opportunity’ context, using self-reported smoking allowance and cigarette availability. We show its clinical utility by establishing its association with smoking occurrences using Granger causality. Next, we mine several informative features from GPS traces, including the novel location context of smoking spots, to develop the SmokingOpp model for automatically detecting the smoking ‘opportunity’ context. Finally, we train and evaluate the SmokingOpp model using 15 million GPS points and 3,432 self-reports from 90 newly abstinent smokers in a smoking cessation study.
In this paper, we define the general concept of ‘opportunity’ contexts and apply it to the case of smoking cessation. We mine several informative features from GPS traces, including the novel location context of smoking spots, to develop the SmokingOpp model for automatically detecting the smoking ‘opportunity’ context.
Contemporary Clinical Trials
engagement, Micro-randomized trial (MRT), mobile health (mHealth), self-regulatory strategies, smoking cessation
November 2021
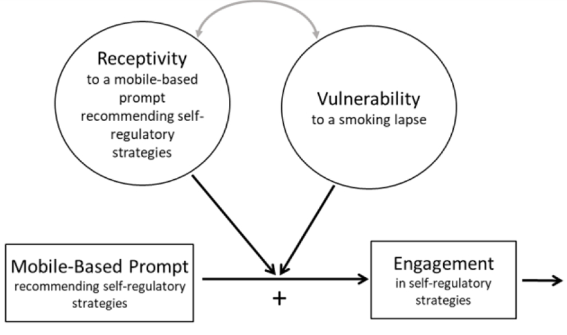
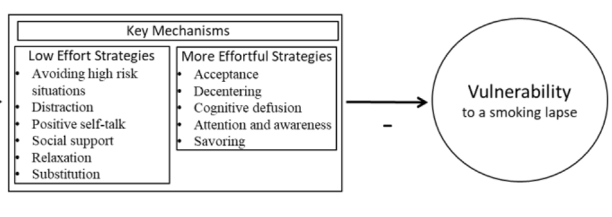
Smoking is the leading preventable cause of death and disability in the U.S. Empirical evidence suggests that engaging in evidence-based self-regulatory strategies (e.g., behavioral substitution, mindful attention) can improve smokers’ ability to resist craving and build self-regulatory skills. However, poor engagement represents a major barrier to maximizing the impact of self-regulatory strategies. This paper describes the protocol for Mobile Assistance for Regulating Smoking (MARS) – a research study designed to inform the development of a mobile health (mHealth) intervention for promoting real-time, real-world engagement in evidence-based self-regulatory strategies. The study will employ a 10-day Micro-Randomized Trial (MRT) enrolling 112 smokers attempting to quit. Utilizing a mobile smoking cessation app, the MRT will randomize each individual multiple times per day to either: (a) no intervention prompt; (b) a prompt recommending brief (low effort) cognitive and/or behavioral self-regulatory strategies; or (c) a prompt recommending more effortful cognitive or mindfulness-based strategies. Prompts will be delivered via push notifications from the MARS mobile app. The goal is to investigate whether, what type of, and under what conditions prompting the individual to engage in self-regulatory strategies increases engagement. The results will build the empirical foundation necessary to develop a mHealth intervention that effectively utilizes intensive longitudinal self-report and sensor-based assessments of emotions, context and other factors to engage an individual in the type of self-regulatory activity that would be most beneficial given their real-time, real-world circumstances. This type of mHealth intervention holds enormous potential to expand the reach and impact of smoking cessation treatments.
This paper describes the protocol for Mobile Assistance for Regulating Smoking (MARS) – a research study designed to inform the development of a mobile health (mHealth) intervention for promoting real-time, real-world engagement in evidence-based self-regulatory strategies.
You must be logged in to post a comment.
No Comments