The mDOT Center
Transforming health and wellness via temporally-precise mHealth interventions






mDOT@MD2K.org
901.678.1526
901.678.1526








Collaborating Investigators:
Dr. Bonnie Spring (CO-PI), Northwestern University
Dr. Inbal Nahum-Shani, University of Michigan
Funding Status:
NIH/NIDDK
2016 – 2021
Associated with:
Contemporary Clinical Trials, Vol 109
October 1, 2021
micro-randomized trial,
optimization, mHealth,
smoking, stress,
digital intervention,
just-in-time
adaptive intervention.
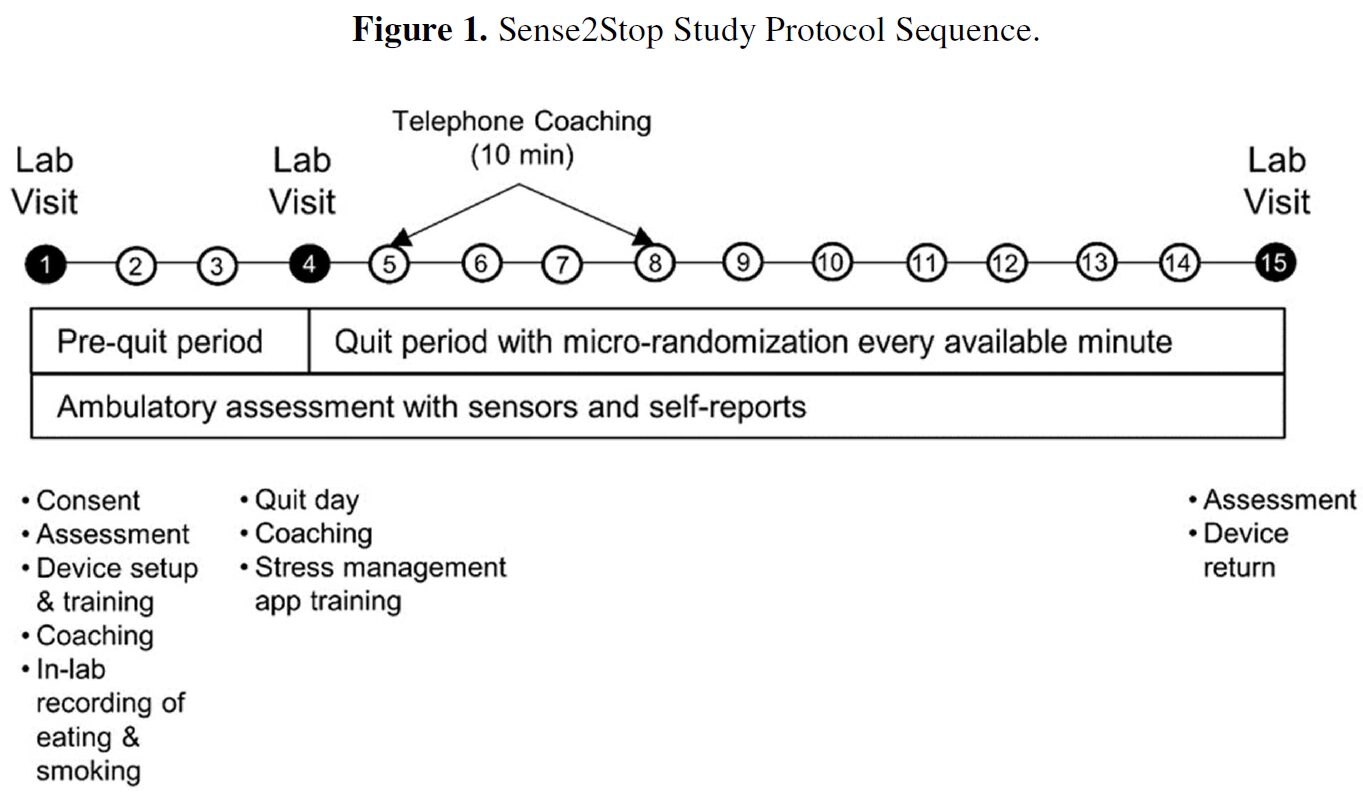
This publication describes Sense2Stop, a micro-randomized trial (MRT) designed to optimize a just-in-time adaptive intervention (JITAI) for preventing smoking relapse. The study uses wearable sensors to objectively detect real-time stress levels in adult smokers. When stress is detected, the smartphone app may deliver a prompt to perform stress management exercises via specialized apps (Mood Surfing, Thought Shakeup, Headspace). The trial aims to determine if intervention prompts reduce stress and smoking likelihood in the subsequent two hours, and whether existing stress moderates these effects, ultimately seeking to enhance treatment efficacy by adapting intervention timing.


Bonnie Spring, Inbal Nahum-Shani, Samuel L Battalio, David E Conroy, Walter Dempsey, Elyse Daly, Sara A Hoffman, Timothy Hnat, Susan Murphy, Marianne Menictas, Shahin Samiei, Tianchen Qian, Jamie Yapp, Santosh Kumar,
ANNALS OF BEHAVIORAL MEDICINE, Vol 57
April 1, 2023
About this code
This example code calculates the minimum sample size for Sense2Stop, a 10-day, stratified micro-randomized trial. Study participants wear a chest and wrist band sensors that are used to construct a binary, time-varying stress classification at each minute of the day. The intervention is a smartphone notification to remind the participant to access a smartphone app and practice stress-reduction exercises. Intervention delivery is constrained to limit participant burden (a limit on the number of reminders sent) and to times at which the sensor-based stress classification is possible. The trial was designed to answer the questions: “Is there an effect of the reminder on near-term, proximal stress if the individual is currently experiencing stress? And, does the effect of the reminder vary with time in study?”
How can a behavioral scientist use this code?
Behavioral intervention scientists can use this code as a starting point for calculating the sample size and power for micro-randomized trials that are similar to the Sense2Stop study.
What method does this code implement?
The code is based on a novel method that (i) balances small sample bias and power, (ii) avoids causal bias, and (iii) informs the expected number of episodes during the remaining part of the day that will be classified as stressed versus not stressed using a generative model (that is based on prior observational study data). Note that the code was developed to be specific to the design of the Sense2Stop study. Significant modification may potentially be needed should this code be used as a starting point to calculate sample size in studies whose design have significant departures from the Sense2Stop study design.
Obesity’s high prevalence and costs make it a public health crisis, but standard of care treatment impedes uptake and depletes resources by taking a one-size-fits-all approach. Guidelines recommend provision of expensive, burdensome treatment components (e.g., counseling, meal replacement) continuously to all consumers regardless of weight loss response. Stepped care that tries less costly evidence-based treatments first, reserving more resource-intensive treatments for suboptimal responders is a logical, equitable population health management strategy. However, stepped care approaches to obesity treatment have not yet incorporated inexpensive, widely available mHealth tools. The potential pitfall of beginning with mHealth treatment is that long-term outcome may be poor if nonresponse to initially insufficient treatment allows demoralization to set in. To reduce that risk, SP4 identifies nonresponders earlier than previously has been possible by applying a predictive model derived from its prior mHealth obesity research and quickly reallocates nonresponders to augmented treatment. The overall objective of this study is to determine the best way to sequence the delivery of mHealth tools and traditional treatment components in a stepped program of obesity treatments. By sequentially delivering treatment components based on participant response and by the use of an innovative experimental approach, the Sequential Multiple Assignment Randomized Trial, this study permits achievement of the target outcome, weight loss, with least resource consumption and participant burden.
SP4 is interested in the personalization algorithms proposed by TR&D2, in particular the use of these stochastic algorithms to determine the randomization probabilities in the MRT. If the TR&D algorithms under Aims 1 and 2 are demonstrated to be robust, SP4 would be interested in including extra participants so as to conduct a feasibility study for use in informing SP4’s future research.
You must be logged in to post a comment.
No Comments